All Articles

Tools and Resources
Drug Updates
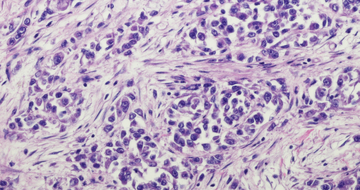
Pharmacist's Application
to Practice: Nogapendekin
alfa inbakicept-pmln
Devan Parker, PharmD, PGY2 Oncology Pharmacy Resident and Juliana Leedy, PharmD, MS, Clinical Oncology Pharmacist, The University of Alabama at Birmingham (UAB) Hospital, Birmingham, Alabama writes about nogapendekin alfa inbakicept-pmln.

Journal Club
Trifluridine–Tipiracil and Bevacizumab in Refractory Metastatic Colorectal Cancer
The data from this trial confirm that FTD–TPI plus bevacizumab is an effective treatment option for patients with refractory metastatic colorectal cancer, irrespective of mutational status, which side the tumor is on, and whether patients have previously