Glofitamab (Columvi) for relapsed or refractory Diffuse Large B-Cell Lymphoma (DLBCL) after at least two previous lines of therapy. Corresponding FDA Drug Update: FDA grants accelerated approval to glofitamab-gxbm for selected relapsed or refractory large B-cell lymphomas | FDA
What is the potential role for glofitamab in the treatment of relapsed or refractory DLBCL?
- Glofitamab is a Bispecific T-Cell Engager that targets both CD20 on B cells and CD3 on T cells, which results in T-cell activation/proliferation, cytokine secretion, and lysis of CD20-expressing B cells1
- Glofitamab is currently recommended in the NCCN guidelines as a third-line option for T-cell Mediated Therapy in patients with DLBCL2
- Guidelines also recommend potential use of similar medications in the same setting including loncastuximab tesirine, polatuzumab vedotin, and epcoritamab
- Currently there are only Phase I/II studies published evaluating the safety and efficacy of glofitamab3
- Baseline characteristics: median age 66 years, 65% male, 71% with DLBCL diagnosis, 60% of patients previously received > 3 previous lines of therapy
- Primary endpoint of Independent Review Committee-assessed Complete Response (CR) was met in 39% of patients
- Median Progression-Free Survival (PFS) of 5 months
- Overall Survival (OS) at 18-month follow-up was 41%
- Only 9% of patients experienced adverse events that led to glofitamab discontinuation
- There are currently no direct head-to-head comparisons of glofitamab with epcoritamab, another Bispecific T-Cell Engager targeting CD20 and CD3 that is FDA-approved for relapsed or refractory DLBCL
- Phase I/II studies evaluating epcoritamab found similar response rates with an Overall Response Rate (ORR) of 63.1% with a CR rate 38.9%4
- Epcoritamab requires more injections based off dosing schedule and is not considered a fixed duration treatment, unlike glofitamab that is a fixed duration schedule of only 12 injections
What role can the pharmacist play in the management of patients on glofitamab?1
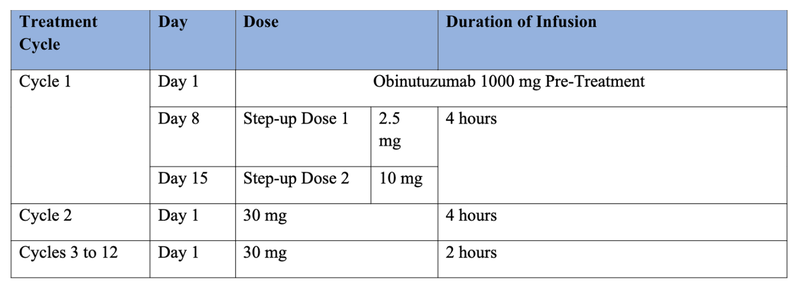
- All patients should be pretreated with a single dose of 1000 mg Obinutuzumab as an IV infusion on C1D1 for Cytokine Release Syndrome (CRS) prevention
- The obinutuzumab is only given during Cycle 1 – patients will only receive the glofitamab with standard pre-medications during future cycles
- Pre-medications for glofitamab infusions include IV dexamethasone, PO/IV antihistamine, and PO acetaminophen
- After Cycle 3, IV dexamethasone is only given in patients who experienced CRS during previous cycles
- Recommended Glofitamab Dosing Schedule (21-day treatment cycles):
- The cytokine release seen with glofitamab may suppress the activity of CYP enzymes, resulting in increased exposure to CYP substrates. This is more likely to occur after C1D8, C2D1, and during/after CRS
- No studies evaluating drug interaction potential have been conducted at this time
- Recommended prophylaxis:
- Consider use of anti-hyperuricemic agents and adequate hydration to patients at risk for Tumor Lysis Syndrome
- Consider antiviral prophylaxis to prevent HSV/VZV reactivation
- Consider prophylaxis for Pneumocystic jirovecii pneumonia in patients prior to administration
- Most frequent adverse events include CRS (70%), musculoskeletal pain (21%), fatigue (20%), and rash (20%)
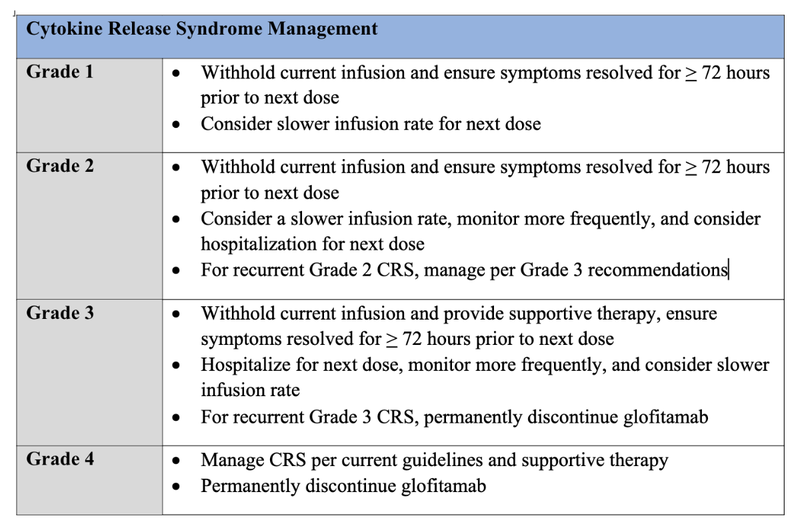
Cytokine Release Syndrome Management
Grade 1
- Withhold current infusion and ensure symptoms resolved for > 72 hours prior to next dose
- Consider slower infusion rate for next dose
Grade 2
- Withhold current infusion and ensure symptoms resolved for > 72 hours prior to next dose
- Consider a slower infusion rate, monitor more frequently, and consider hospitalization for next dose
- For recurrent Grade 2 CRS, manage per Grade 3 recommendations
Grade 3
- Withhold current infusion and provide supportive therapy, ensure symptoms resolved for > 72 hours prior to next dose
- Hospitalize for next dose, monitor more frequently, and consider slower infusion rate
- For recurrent Grade 3 CRS, permanently discontinue glofitamab
Grade 4
- Manage CRS per current guidelines and supportive therapy
- Permanently discontinue glofitamab
- For patients with commercial insurance, the Genentech Oncology Co-pay Assistance Program is available which could provide up to $25,000 of co-pay assistance per year. For patients with no insurance or if income meets specific criteria, the Genentech Patient Foundation Manufacturer’s Assistance program is available.5
Clinical Pearls1
- Glofitamab is available in a 1 mg/1 mL solution that is injected into either sodium chloride 0.9% or 0.45% with a desired final concentration of 0.1-0.6 mg/mL. It is only compatible with infusion bags made of PVC.
- Vials should be stored at 2-8°C and should not be frozen or shaken. After solution has been diluted, it should be used immediately.
- No differences observed in patients based on mild/moderate renal impairment, mild hepatic impairment, gender, or body weight.
- No differences in efficacy based on age, however there were higher rates of adverse events seen in patients > 65 years of age (primarily based on COVID-19 infection).
- There are currently no data available on glofitamab use in patients with severe renal impairment/end-stage renal disease, moderate or severe hepatic impairment, or in pediatric patients.
- There is no data available in pregnant patients, however based on the mechanism of action it is recommended to not become pregnant on this medication and to use effective contraception during treatment and for one month after the last dose.
- Women should not breastfeed while on treatment or for one month after the last dose.
References
1.COLUMVI [package insert]. South San Franscisco, CA; Genentech Inc; 2023.
2.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: B-Cell Lymphomas. Version 6, 2023. Accessed October 16, 2023. https://www.nccn.org/professionals/physicians_gls/pdf/b-cell.pdf
3.Dickinson MJ, Carlo-Stella C, Morschhauser F, et al. Glofitamab for Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med. 2022;387(24):2220-2231. doi:10.1056/NEJMoa2206913
4.Thieblemont C, Phillips T, Ghesquieres H, et al. Epcoritamab, a Novel, Subcutaneous CD3xCD20 Bispecific T-Cell-Engaging Antibody, in Relapsed or Refractory Large B-Cell Lymphoma: Dose Expansion in a Phase I/II Trial. J Clin Oncol. 2023;41(12):2238-2247. doi:10.1200/JCO.22.01725
5.Financial Assistance Options | COLUMVITM (glofitamab-gxbm). columvi. Accessed October 16, 2023. https://www.columvi.com/financial-support/assistance-options.html