FDA Approval date: August 14, 2023
FDA Alert: FDA Approves Melphalan Liver Directed Treatment Uveal Melanoma
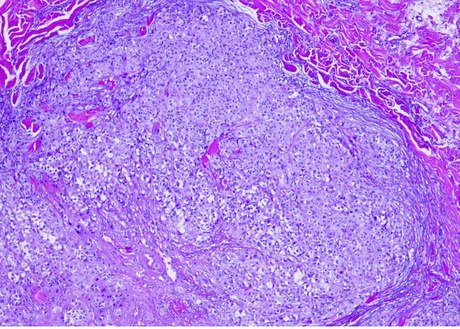
Hepzato Kit™ (melphalan) for Injection/Hepatic Delivery System (HDS) is FDA approved as a liver-directed therapy for treatment of adult patients with uveal melanoma with unresectable hepatic metastasis affecting less than 50% of the liver and no extrahepatic disease, or extrahepatic disease limited to the bone, lymph nodes, subcutaneous tissues, or lung that is amenable to resection or radiation.
What is the potential role for Hepzato Kit™ in the treatment of uveal melanoma?
- Ocular melanomas represent approximately up to 5% of all melanomas in the United States (U.S.), with 85% of those reported to be uveal melanoma. Uveal melanoma is considered to be rare, with an estimated incidence of 2000 cases per year in the U.S.; however, it is associated with relatively high mortality secondary to metastasis.1-2
- Despite excellent rates of initial local disease control, over 50% of patients will experience disease recurrence or metastasis within 3-5 years.
- An estimated 90% of these metastases are concentrated predominantly within the liver or are solely confined to it.
- Metastatic uveal melanoma has an associated poor prognosis with a mean overall survival (OS) of approximately 1 year. Additionally, treatment options at this stage are limited and are often extrapolated from cutaneous melanoma therapies despite the routine exclusion of patients with uveal melanoma from clinical trials.
- Prior to the approval of the Hepzato Kit™, the only treatment option for metastatic uveal melanoma was tebentafusp-tebn (Kimmtrak®), a bispecific glycoprotein 100 (gp100) peptide-human leukocyte antigen (HLA)-directed CD3 T-cell engager indicated as a systemic treatment for HLA-A*02:01-positive adult patients with unresectable or metastatic uveal melanoma. However, only approximately 45% of people in the United States are HLA-A*02:01-positive, leaving the other 55% of patients with no FDA-approved systemic options.3
- The Hepzato Kit™ (melphalan/hepatic delivery system [HDS]) is the only liver-directed therapy approved by the FDA for the treatment of metastatic uveal melanoma.
- Hepzato Kit is approved for patients with uveal melanoma with hepatic metastasis that are unresectable and affect less than 50% of the liver and no or limited extrahepatic disease.4
- Hepzato (melphalan), a component of the Hepzato kit™, is an alkylating drug administered via an intra-arterial infusion into the hepatic artery.4
- Administration of Hepzato requires general anesthesia and extracorporeal bypass of circulation.4
- The use of the HDS is a percutaneous hepatic perfusion (PHP) procedure utilizing a double-balloon catheter percutaneously inserted into the inferior vena cava to isolate hepatic venous blood flow. This allows isolation of liver circulation allowing for direct delivery of highly concentrated chemotherapy loco-regionally, as blood exits the liver the specialized filters remove approximately 85% of the chemotherapy from the blood which in turn limits systemic toxicities.4
- FDA approval of the Hepzato Kit™ was based on the FOCUS study, a phase 3, randomized study (Trial 301) designed to evaluate outcomes of PHP with the Hepzato Kit™ versus best alternative care (BAC). However, due to challenges encountered in patient enrollment, the trial underwent modification through an amendment, leading to the cessation of the BAC arm. The resulting single arm, multicenter, open-label trial (Trial 301A) reported on a total of 91 patients with uveal melanoma with unresectable hepatic metastases with limited extrahepatic disease in the bone, subcutaneous sites, lymph nodes, or lung on the contingency that the hepatic metastasis were the life-threatening metastasis.5
- Patients assigned to PHP (Trial 301: n = 40; 301A: n = 51) received Hepzato Kit via intra-arterial infusion at a dose of 3 mg/kg based on ideal body weight (max 220 mg per single Hepzato treatment), infused over 30 minutes, followed by a 30-minute washout period. Treatments were administered every 6 to 8 weeks, for up to six treatments (average of four treatments).5
- Patients were excluded if they had metastases in greater than/equal to 50% of the liver parenchyma, Child-Pugh Class B or C cirrhosis, or hepatitis B or C infection, or ECOG greater than 2.5
- Objective response rate (ORR) was 36.3% (95% CI: 26.4, 47) with a complete response (CR) in 7.7% of patients and a partial response (PR) in 28.6% of patients.5
- Median duration of response (DoR) was 14 months (95% CI: 8.3, 17.7), with 30% of responders had a DoR > 12 months.5
- The most common adverse reactions and lab abnormalities included: myelosuppression (Grade 3/4: 68%), thrombocytopenia (65%; grade 3/4: 55%), fatigue (65%), anemia (63%; grade 3/4: 33%), nausea (57%), musculoskeletal pain (46%), leukopenia (46%), abdominal pain (39%), neutropenia (35%; grade 3/4: 33%), vomiting (35%), increased alanine aminotransferase (ALT) (32%), prolonged activated partial thromboplastin time (aPTT) (28%), increased aspartate aminotransferase (AST) (28%), increased alkaline phosphatase (27%), and dyspnea (23%).5
- Hepzato is contraindicated in patients with:4
- Active intracranial metastasis or brain lesions with a propensity to bleed;
- Liver failure, portal hypertension, or known varices at risk for bleeding;
- Surgery or medical treatment of the liver in the previous 4 weeks;
- Uncorrectable coagulopathy;
- Inability to safely undergo general anesthesia;
- History of allergy or known hypersensitivity to melphalan or any components in the Hepzato Kit™, including: natural rubber latex, heparin or presence of heparin-induced thrombocytopenia (HIT), or iodinated contrast not controlled by premedication.
What role can the pharmacist play in the management of patients treated with Hepzato Kit?4
- Due to the complexity of Hepzato Kit™ administration and potential for significant complications during the PHP procedure, such as bleeding, damage to liver cells, and clot-related events, access to the Hepzato Kit™ will be limited to facilities who enroll in controlled program known as the Hepzato Kit™ Risk Evaluation and Mitigation Strategy (REMS).
- Pharmacist involvement with policy, procedure, and program development will be vital to assure safety in drug ordering, dosing, administration, storage and monitoring.
- Hepzato is administered intra-arterially using the Hepatic Delivery System (HDS) every 6-8 weeks but can be delayed until recovery from toxicities and as per clinical judgement, for up to 6 infusions.
- Hepzato (melphalan) is dosed based on ideal body weight (IBW) at a dose of 3 mg/kg with a maximum dose of 220 mg.
- Hepzato Kit dosing utilizes an uncommon IBW calculation which differs from the common/accepted formula in the U.S. Incorporation of the correct IBW calculation and associated Hepzato dosing will be an important consideration when implementing a Hepzato Kit dosing protocol or electronic medication order.
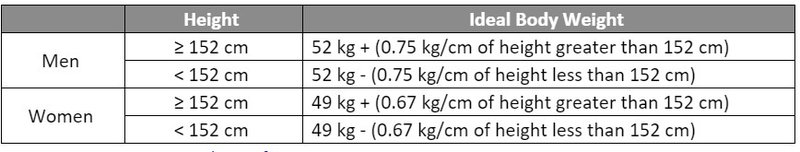
Source: Hepzato Kit Prescribing Information.
- Dose modifications:
- A dosage reduction to 2 mg/kg is recommended for subsequent infusions for grade 4 neutropenia or thrombocytopenia.
- Hepzato Kit should only be administered in patients with platelets >100,000/μL, hemoglobin ≥10 gm/dL, and neutrophils >2,000/μL.
- Platelets and clotting factors may be removed during the Hepzato Kit procedure.
- Discontinue drugs affecting platelet function such as aspirin, nonsteroidal anti-inflammatory drugs, or other anti-platelet drugs one week before the procedure.
- Procedure-related reductions in blood pressure including severe hypotension can occur.
- To reduce the risk of severe hypotension, assess hypothalamic-pituitary-adrenal axis function, and temporarily discontinue ACE-inhibitors, calcium channel blockers, or alpha-1-adrenergic blockers for at least 5 half-lives prior to procedure.
Clinical Peals4
- Administration of Hepzato via the Hepzato Kit™ is only approved in patients weighing 35 kg or greater due to potential size limitations with respect to percutaneous catheterization.
- The double balloon catheter component of the HDS contains natural rubber latex which may cause allergic reactions in patients with a latex allergy.
- Ensure the patient is euvolemic but do not overhydrate the patient.
- Monitor for peri-procedural complications during and for at least 72 hours following the procedure.
- To mitigate the risk of thromboembolic events, administer anticoagulation as per manufacturer specific recommendations during the procedure.
- Due to the risk of bleeding, do not use in patients with uncorrectable coagulopathies and delay treatment with the Hepzato Kit for at least 4 weeks after surgery or other medical procedure involving the liver.
- In Europe, Hepzato is a standalone medical device, approved in 2012, under the trade name Chemosat Hepatic Delivery System for Melphalan, or Chemosat, and has been used at major medical centers to treat a wide range of cancers of the liver.
References
1.Aronow ME, Topham AK, Singh AD. Uveal Melanoma: 5-Year Update on Incidence, Treatment, and Survival (SEER 1973-2013). Ocul Oncol Pathol. 2018 Apr;4(3):145-151. doi: 10.1159/000480640.
2.Krantz BA, et al. Uveal melanoma: epidemiology, etiology, and treatment of primary disease. Clin Ophthalmol. 2017;11:279–289. doi:10.2147/OPTH.S89591
3.Bai H, Bosch JJ, Heindl LM. Current management of uveal melanoma: A review. Clin Exp Ophthalmol. 2023 Jul;51(5):484-494. doi: 10.1111/ceo.14214.
4.Hepzato Kit. Delcath: Corporate Presentation. September 6, 2023. https://delcathsystemsinc.gcs-web.com/static-files/2c090a2f-60b0-4c1f-9a7d-ac45617a0ae3
5.Zager JS, et al. FOCUS phase 3 trial results: Percutaneous hepatic perfusion (PHP) with melphalan for patients with ocular melanoma liver metastases (PHP-OCM-301/301A). J Clin Oncol. 2022;40(16):9510–9510. doi:10.1200/JCO.2022.40.16_suppl.9510