Quizartinib for Acute Myeloid Leukemia (AML) with FLT3 internal tandem duplication mutationCorresponding FDA Drug Update on July 20, 2023: FDA approves quizartinib for newly diagnosed acute myeloid leukemia
What is the potential role for quizartinib in the treatment of acute myeloid leukemia (AML)?
- Quizartinib is a FLT3 receptor tyrosine kinase inhibitor that blocks FLT3 internal tandem duplication (FLT3-ITD) dependent cell proliferation.1
- Quizartinib is the first-line treatment of adults with newly diagnosed acute myeloid leukemia (AML) that is FLT3-ITD positive in combination with standard AML chemotherapy, cytarabine and anthracycline induction and cytarabine consolidation, and as maintenance therapy alone post consolidation.1
- Quizartinib was approved based on the QuANTUM-First trial.2
- Phase 3 trial evaluating the efficacy and safety of quizartinib versus placebo in combination with induction and consolidation chemotherapy in AML
- Efficacy evaluated as overall survival (OS) after minimum follow-up of 24 months
- Quizartinib had longer median OS of 32 months versus 15 months with placebo (HR 0.78, 95% CI 0.62-0.98)
- Other medications targeting FLT3 mutated AML include midostaurin and gilteritinib.3
- Midostaurin has activity against both FLT3 ITD and tyrosine kinase domain (TKD) alterations, but is less potent than quizartinib against FLT3 ITD.
- Midostaurin is also approved in the front-line setting in combination with induction and consolidation chemotherapy based on the results of the RATIFY trial in 2017.
- Midostaurin had longer median OS of 75 months versus 26 months with placebo at a median follow up of 59 months (HR 0.78, 95% CI 0.63-0.96).4
- Gilteritinib is approved in the relapsed/refractory setting only.5
- Compared to the RATIFY study, patients included in the QuANTUM-First trial were older (median age 56 versus 48 years old). Since FLT3-ITD alterations carry a worse prognosis compared to FLT3-TKD alterations, the inclusion of 22.6% of patients with TKD alterations in the RATIFY study could explain the longer OS observed in that cohort.
- Although older patients were included in QuANTUM-First, a post-hoc subgroup analysis did not support significant improvement in OS in patients >60 years old (unstratified HR 0.91; 95% CI 0.66-1.26).
- Quizartinib in the QuANTUM-First trial showed greater relapse-free survival rate (39.3% vs. 13.6%, HR 0.61, 95% CI 0.44-0.85) and less incidence of relapse at 12 months than placebo (19% (95% CI 12.7-25.6) vs. 35% (95% CI 27.1-42.7)).2,3,4
- The use of quizartinib as the first-line FLT3 inhibitor may be appealing as the majority of resistance arises due to development of FLT3 TKD alterations, which may be sensitive to type 1 FLT3 inhibitors, such as midostaurin.6
- Quizartinib is a category 1 recommended option for induction eligible and category 2B option for maintenance AML with FLT3-ITD mutation treatment in the National Comprehensive Cancer Network (NCCN) guidelines.7
What role can the pharmacist play in the management of patients on quizartinib?
- Pharmacists can play a key role in quizartinib management including dosing, monitoring, storage, drug interactions, and side effect control.1
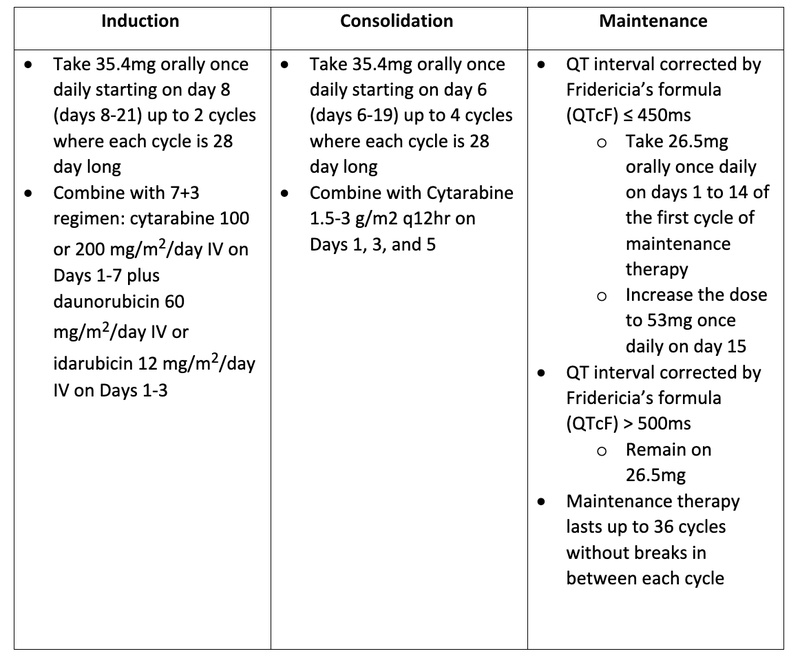
- Dosing
- Electrocardiogram (ECG) Monitoring
- Perform patient’s electrocardiogram (ECG) before starting the dose during induction and consolidation phases. Monitor ECG once weekly during the quizartinib treatment.
- Take 35.4mg orally once daily starting on day 8 (days 8-21) up to 2 cycles where each cycle is 28 day long
- Combine with 7+3 regimen: cytarabine 100 or 200 mg/m2/day IV on Days 1-7 plus daunorubicin 60 mg/m2/day IV or idarubicin 12 mg/m2/day IV on Days 1-3
- Take 35.4mg orally once daily starting on day 6 (days 6-19) up to 4 cycles where each cycle is 28 day long
- Combine with Cytarabine 1.5-3 g/m2 q12hr on Days 1, 3, and 5
- QT interval corrected by Fridericia’s formula (QTcF) ≤ 450ms
- Take 26.5mg orally once daily on days 1 to 14 of the first cycle of maintenance therapy
- Increase the dose to 53mg once daily on day 15
- QT interval corrected by Fridericia’s formula (QTcF) > 500ms
- Remain on 26.5mg
- Maintenance therapy lasts up to 36 cycles without breaks in between each cycle
- Electrocardiogram (ECG) Monitoring
- Perform patient’s electrocardiogram (ECG) before starting the dose during induction and consolidation phases. Monitor ECG once weekly during the quizartinib treatment.
- Take patient’s ECG before starting maintenance phase, once a week for at least the first month after initiating and escalating dose, and as needed thereafter as clinically indicated.
- Quizartinib should be taken at the same time each day with or without food. Wait until the next planned dose of quizartinib on the following day if patient vomits after taking a dose of quizartinib.
- CYP3A4 Drug Interactions
- Reduce quizartinib dose if taking with strong CYP3A4 inhibitors
- If current dose is 53mg/day, modify to 26.5mg/day
- If current dose is 35.4mg/day, modify to 17.7mg/day
- If current dose is 26.5mg/day, modify to 17.7mg/day
- If current dose is 17.7mg/day, halt quizartinib for the strong CYP3A4 inhibitor duration and resume quizartinib after discontinuing CYP3A4 inhibitor for five half lives
- Avoid quizartinib if taking with strong or moderate CYP3A4 inducers
- The most common side effects (>20%) associated with quizartinib include diarrhea, nausea, abdominal pain, headache, mucositis, and febrile neutropenia.
- Quizartinib has a black box warning for QT interval prolongation.
- QT prolongation can cause dizziness, lightheadedness, and fainting
- 10% of patients had an increased from baseline QTcF of greater than 60ms
- More serious grade 4 adverse events include Torsades de pointes (0.2%), polymorphic ventricular tachycardia, and life-threatening arrhythmia.
- Permanently discontinue quizartinib if patient experiences these side effects
- Daiichi Sankyo Access Central offers quizartinib patient assistance program.8
- Eligibility requirements are the following:
- Currently live in the United States
- No coverage under any private or public insurance
- An annual income equal to or lower than a certain level
- Not qualified or enrolled in Medicare Part D Low Income Subsidy (LIS)
- Have used at least 3% of annual household income on prescription drugs this year
Clinical Pearls
- Storage1
- At room temperature between 20 to 25 degrees Celsius (68 to 77 Fahrenheit).
- Approved tests/diagnostics9
- Presence of FLT3-ITD mutation is detected by LeukoStrat CDx FLT3 Mutation Assay.
- Risk Evaluation Mitigation Strategy (REMS) Program1,10
- Quizartinib is only available through REMS that encourages patients to carry a patient wallet card which lists the signs and symptoms related to QT prolongation.
- REMS certification is required for all pharmacies and prescribers in order to supply quizartinib.
- The authorized representative must enroll the pharmacy, finish the necessary training, ensure that the policies and procedures put in place to execute the REMS standards are followed, and make sure that staff is trained and complied with the REMS requirements
References
1.VANFLYTA (quizartinib). FDA Package Insert. Accessed October 15, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/216993s000lbl.pdf
2.Erba P, Montesinos P, Kim H, et al. Quizartinib plus chemotherapy in newly diagnosed patients with FLT3-internal-tandem-duplication-positive acute myeloid leukaemia (QuANTUM-FIRST): a randomized, double-blind, placebo-controlled, phase 3 trial. LANCET. 2023;401(10388):P1571-1583.
3.Phillips C. Quizartinib Approval Adds New Treatment Option for AML, Including in Older Patients. NIH National Cancer Institute. https://www.cancer.gov/news-events/cancer-currents-blog/2023/fda-vanflyta-aml-flt3 Accessed October 15, 2023.
4.Stone R, Mandrekar S, Sanford B, et al. Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation. NEJM. 2017;377:454-464.
5.XOSPATA (gilteritinib). FDA Package Insert. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/211349s000lbl.pdf Accessed October 15, 2023.
6.Emery Dylann. OncLive. Quizartinib Plus Induction Chemotherapy Provides Survival Benefit in FLT3 ITD-Mutated AML. https://www.onclive.com/view/quizartinib-plus-induction-chemotherapy-provides-survival-benefit-in-flt3-itd-mutated-aml Accessed October 15, 2023.
7.Acute Myeloid Leukemia. NCCN Guidelines. Version 5. 2023. https://www.nccn.org/professionals/physician_gls/pdf/aml.pdf Accessed October 15, 2023.
8.VANFLYTA Patient Assistance Program. Daiichi-Sankyo Access Central. https://dsiaccesscentral.com/hcp/vanflyta/financial-help/pap Accessed October 15, 2023.
9.List of Cleared or Approved Companion Diagnostic Devices. FDA. https://www.fda.gov/medical-devices/in-vitro-diagnostics/list-cleared-or-approved-companion-diagnostic-devices-in-vitro-and-imaging-tools Accessed October 15, 2023.
10.Roles of Different Participants in REMS. FDA. https://www.fda.gov/drugs/risk-evaluation-and-mitigation-strategies-rems/roles-different-participants-rems#:~:text=Pharmacists%20play%20a%20key%20role,in%20place%20prior%20to%20dispensing. Accessed October 15, 2023.